Our Products
UPT (UTP-3A) Lp-PLA2 Kit
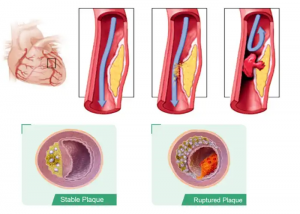
UPT (UTP-3A)® Lp-PLA2 Kit Test Principle
Up-converting Phosphor Technology
UPT (UTP-3A)® Lp-PLA2 Kit Features
1. Fully quantitative measurement
2. Rapid, accurate, lab quality results within minutes
3. Ready-to-use reagents
4. Serum, plasma are applicable as sample
5. Long shelf life
6. Easy storage at room temperature
7. Single dose format: run only the test you need
UPT (UTP-3A)® Lp-PLA2 is an accurate, rapid, fully quantitative test used on UPT (UTP-3A)® Reader for the determination of Lipoprotein Associated Phospholipase A2 (Lp-PLA2) in human serum and plasma as a risk marker for cardiovascular disease.
Lp-PLA2: the independent risk marker for cardiovascular disease.
Lipoprotein-associated phospholipase A2 (Lp-PLA2) is also known as platelet-activating factor acetylhydrolase (PAF-AH). Lp-PLA2 is an enzyme that appears to play a role in the inflammation of blood vessels and is thought to help promote atherosclerosis.
Some recent studies have shown that Lp-PLA2 is an independent risk marker for cardiovascular disease (CVD), including coronary heart disease (CHD), and ischemic stroke. The Lp-PLA2 test is thus used to help evaluate a person’s risk of developing coronary heart disease (CHD) or to help determine the risk of having an ischemic stroke.
The test would typically be used to evaluate an individual who is at a moderate to elevated risk for CHD or stroke, someone with one or more other risk factors. For instance, it may be ordered when someone has normal or minimally elevated lipid levels, borderline high blood pressure (hypertension), or metabolic syndrome.
An Lp-PLA2 test may sometimes be used along with an hs-CRP test to evaluate a person’s level of underlying inflammation associated with CVD risk. However, unlike hs-CRP, the Lp-PLA2 test is not affected by conditions other than CVD that can cause general inflammation, so it may be used when someone has an inflammatory condition, such as arthritis.
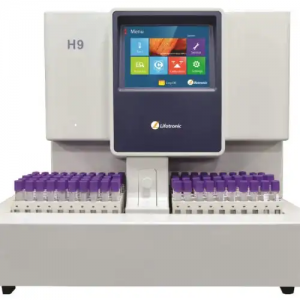
H9- HbA1C ANALYZER (HPLC)
lowest consumable prices )
H9 HBA1C ANALYZER (HPLC)
Gold Standard Of Diabetes Diagnose
HPLC Methodology
H9 Hemoglobin Analyzer(HPLC)
HPLC Technology(Gold Standard Methodology)
1. fast- To Ensure Your Workload is Processed Promptly
2. Fully Automated – To Minimize Operation Hassles
3. Precise and Reliable – To Serve You Consitently.
1. User Friendly- To Avoid Operator Error
2. Flexible- To Satisfy Diverse Laboratory’s Needs
3. Complex System Functions- For Easy integration
4. Compact Size- To Minimize Space Requirements
Specification
METHODOLOGY :- High-Pressure Liquid Chromatography (HPLC)
TEST MODES :- Fast Mode, Variant Mode, thalassemia Mode
TEST RANGE :- 3% — 18%
PRECISION :- CV < 1 %
FIRST SAMPLE RESULT :- 2.4 Mins
TEST SPEED :- 1 .2Mins/ sample
SAMPLE TYPE :- Venous Blood, Finger Peripheral Blood, Lyophilized Whole Blood
SAMPLE VOLUME :- 5ul , Peripheral Blood, 3ul (1000 Dilution Rate)
AUTO SAMPLE STATION :- 110 Positions+1 Stat Position
PHOTOMETER :- 415nm+500nm LED, 20000 Hours Life Span
CHROMATOGRAPHY COLUMN :- Available Tests 2 1500T
FILTER :- > 400T
DISPLAY :- 8″TFT True Color LCD Touch Screen
SOFTWARE :- Embedded System with Self-Diagnosis to Monitor and Detect System Errors
REAGENT KIT :- Eluent A, Eluent B, Eluent C, Hemolysin, Calibrator,QC Material
IMFORMATION INPUT :- Scanner or Touch Keypad
STORAGE :- 4000 Sample Results
CONNECTION :- Rs232, USB, LIS Compatible
PRINTER :- Thermal Printer and External Laser Printer
OPERATION TEMPERATURE :- 12~35°C (53~95°F)
HUMIDITY :- 10~ 70%
POWER :- AC 100-240V 50/ 60HZ 150VA
DIMENSIONS :- 580mm x 500mm x 520mm (22.8”H >< 19.7”W x 20.5″D)
WEIGHT :- 50kg (110lbs)
BARCODE SCANNER :- Intemal Automated Scan
NGSP AND IFCC CERTIFIED
POCT ANALYZER (UPT-3A)
Features
Unique “Up-converting Phosphor” Technology.
Luminescence method, Fully quantitative.
Reagent stored at 4~30°C, no need of refrigeration.
Easy Operation.
Rapid, get results in 3~20 minutes.
Multi-item detection.
Single or bulk test(can reach 100 Tests/Hour).
Compact Analyzer
Accurate results can be comparable with the Central Lab.
Support print results directly or upload to the Hospital LIS system.
UPT (UTP-3A) Procalcitonin Kit
80 Procalcitonin is an accurate, rapid, fully quantitative test used on 80 Reader for the determination of the procalcitonin (PCT) in human serum, plasma and whole blood as an aiding tool for diagnosing and controlling the treatment of severe, bacterial infection and sepsis.
Benefits of PCT test in summary:
1. Use as an early and specific bio-marker for severe bacterial infection and sepsis
2. Assess severity of infection and make prognosis
3. Differentiate bacterial from viral and non-infectious inflammation
Guide the application of antibiotics and reduce unnecessary usage of antibiotics
4. Monitor treatment efficacy of antibiotics
80 Procalcitonin Kit Test Principle
Up-converting Phosphor Technology
80 Procalcitonin Kit Features
1. Fully quantitative measurement
2. Rapid, accurate, lab quality results within minutes
3. Ready-to-use reagents
4. Serum, plasma and whole blood are applicable as sample
5. Long shelf life
6. Easy storage at room temperature
7. Single dose format: run only the test you need
(Latest and patented Up-
Converting Phosphor technology)
POCT ANALYZER (UTP-3A)
Principle
up-converting phospher technology (UPT) is a kind of immune detection technology that combine immunochromatographic principle with photoelectric conversion principle. it uses up-converting phosphor particles (ucps) as tracer, after being excited by infrared light,the UCP’s emit visible light, achieving the up-conversion of energy. then get quantitative detection of the test sample rapidly via the conversion of light signals.
PCT: A specific marker of severe bacterial infection and sepsis
Procalcitonin (PCT) is a peptide precursor of the hormone calcitonin, the latter being involved with calcium homeostasis. It is composed of 116 amino acids and is produced by parafollicular cells (C cells) of the thyroid and by the neuroendocrine cells of the lung and the intestine.
The level of PCT in the blood stream of healthy individuals is below the limit of detection (0.01 ng/mL) of clinical assays. The level of procalcitonin rises rapidly (within 6 – 12 hours) in a response to a proinflammatory stimulus, especially of bacterial origin. It does not rise significantly with viral or non-infectious inflammations. Therefore, PCT helps differentiate bacterial from viral infections, the early detection of an elevated PCT level in patients with suspected bacterial infections enabling earlier antibiotic treatment. PCT also supports informed decisions on when to continue or stop antibiotics, improving patient care and decreasing antibiotic misuse and resistance.UPT (UTP-3A) C-Reactive Protein (CRP) Kit
UPT (UTP-3A) Interleukin 6 (IL-6) Kit
UPT (UTP-3A) Lp-PLA2 Kit
UPT (UTP-3A) NT-proBNP Kit
UPT (UTP-3A) Heart-type Fatty Acid Binding Protein (H-FABP) Kit
UPT (UTP-3A) Cardiac Troponin I (cTnI) Kit
UPT (UTP-3A) Myoglobin (MYO) Kit
UPT (UTP-3A) CK-MB Kit
UPT (UTP-3A) D-dimer Kit
UPT (UTP-3A) NGAL Kit
UPT(UTP-3A) Fetal Fibronectin (fFN) Kit
UPT(UTP-3A) Anti-CCP Kit
UPT(UTP-3A) alpha-Fetoprotein (AFP) Kit
UPT(UTP-3A) Golgi Protein 73 (GP73) Kit
UPT(UTP-3A) TIMP-1 Kit
UPT(UTP-3A) PIIINP Kit
UPT(UTP-3A) Collagen IV (CIV) Kit
UPT(UTP-3A) Collagen IV (CIV) Kit
UPT(UTP-3A) Laminin (LN) Kit
UPT(UTP-3A) Hyaluronic Acid (HA) Kit
Allied Biotechnology is the fastest growing Diagnostics Company formed in 2008 with an objective to provide the Stat- Of –The Art Diagnostics instruments and consumables at affordable prices to Indian diagnostic industry.
Screen test for Cardiac Arrest in CVD
NEWLY DEVELOPED LP-PLA2 TEST
Lp-PLA2 test to your LIPID Management Strategy
Cholesterol Alone is NOT Enough!
50% of the patients with a CVD events have normal Cholesterol levels.
A simple Blood test for Lp-PLA2, Uncover the hidden risk of CV Events.
Who Should get the Lp-PLA2 Test?
Lp-PLA2 Test is recommended for patients with established CVD or patients at moderate to intermediate risk for CVD, such as patients with, including but not limited to, two or more of the following risk factors:
Do you know that more than 50% of heart attacks occur in patients with normal cholesterol/ LDL levels?
How does a heart attack occur?
The most common cause of a heart attack is sudden narrowing or blockage of a coronary artery, which blocks oxygen to the heart. This can happen when plaque in the coronary artery breaks and a blood clot forms in the artery.
What is the main cause of heart attacks and Strokes ?
Plaque rupture is the main culprit for acute MI (Heart Attacks) & Sudden Cardiac deaths?
In most adults, cholesterol causes a fatty deposit called plaque to build up in the walls of the arteries.
When these walls become inamed, your body produces an enzyme called Lp-PLA2.
Scientists used to think that narrowing of arteries was the main cause of heart attacks and strokes. However, recent studies show that in over 68% of heart attacks the arteries are not narrow. Instead, the inside wall of the inamed artery becomes weakened and ruptures, letting plaque into the bloodstream, where the plaque can cause a clot.
What is the risk when I have elevated Lp-PLA2 Levels while other tests ( Lipid levels) are normal ?
Individuals who have an elevated Lp-PLA2 level and one or more risk factors have more than twice the risk of having a heart attack. If the Lp-PLA2 is elevated and they have high blood pressure, their risk for stroke increases more than 6 times.
In addition, almost 95% of people with risk factors for heart attack or stroke who have a Lp-Lp-PLA2 value less than 200 ng/ml do not have a heart attack or stroke within 4 years.
What if I have elevated Lp-PLA2 levels?
Therapeutic intervention can lower heart attacks and ischemic stroke in patients with elevated level of Lp-PLA2. Lp-PLA2 levels can be used to identify patients who require more aggressive treatment, including lipid-lowering therapy and lifestyle modication.
Lp-PLA2: the independent risk marker for cardiovascular disease
Lipoprotein-associated phospholipase A2 (Lp-PLA2) is also known as platelet-activating factor acetyl hydrolase (PAF-AH). Lp-PLA2 is an enzyme that appears to play a role in the inflammation of blood vessels and is thought to help promote atherosclerosis.
Some recent studies have shown that Lp-PLA2 is an independent risk marker for cardiovascular disease (CVD), including coronary heart disease (CHD), and ischemic stroke. The Lp-PLA2 test is thus used to help evaluate a person’s risk of developing coronary heart disease (CHD) or to help determine the risk of having an ischemic stroke.
The test would typically be used to evaluate an individual who is at a moderate to elevated risk for CHD or stroke, someone with one or more other risk factors. For instance, it may be ordered when someone has normal or minimally elevated lipid levels, borderline high blood pressure (hypertension), or metabolic syndrome.
Allied Biotechnology is the fastest growing Diagnostics Company formed in 2008 with an objective to provide the Stat- Of –The Art Diagnostics instruments and consumables at affordable prices to Indian diagnostic industry.
CBC-3D
CBC-3D is a tri-level control for monitoring on Automated, Semi-automated and Manual Methods. CBC-3D has 105-day closed vial stability with 14-day open vial stability.
Features
- 105-day closed vial stability; 3 QC months
- 2. 14-day open vial stability
- Packaged in 5mL and 2mL pierceable screw cap tubes and 2mL vials
- Assayed for Abbott CELL-DYN Emerald
CBC-5D MR
CBC-5D is a tri-level control designed specifically for BECKMAN COULTER DxH 800/600, LH700 Series and LH500 hematology analyzers. The assay table includes values for 22 parameters. CBC-5D is bar-coded for correct QC file access. Bar codes are available for uploading assay values. CBC-5D has 105-day closed vial stability with an open vial stability of 14 samples within 14 days.
Features
- 105-day closed vial stability; 3 QC months
- 14 samples within 14 days open vial stability
- Packaged in 5mL pierceable screw cap tubes
- Bar coded label on tubes
CBC-5D MR
CBC-5D is a tri-level control designed specifically for BECKMAN COULTER DxH 800/600, LH700 Series and LH500 hematology analyzers. The assay table includes values for 22 parameters. CBC-5D is bar-coded for correct QC file access. Bar codes are available for uploading assay values. CBC-5D has 105-day closed vial stability with an open vial stability of 14 samples within 14 days.
Features
- 105-day closed vial stability; 3 QC months
- 14 samples within 14 days open vial stability
- Packaged in 5mL pierceable screw cap tubes
- Bar coded label on tubes
CBC-SYS & CBC-X
Features
- 75-day closed vial stability; 2 QC months
- 15 samples within 15 days open vial stability
- Packaged in 4.5mL pierceable screw cap tubes
- Assay CDs available
- Bar coded label on tubes
CBC-3K
CBC-3K is a tri-level control for monitoring Abbott CELL-DYN Sapphire, Ruby and 3200 analyzers and manual methods. Assay tables include values for 20 parameters. Disks are available for uploading assay values. CBC-3K has 75-day closed vial stability with 8-day open vial stability.
Features
- 75-day closed vial stability; 2 QC months
- Packaged in 3mL pierceable screw cap tubes
- Assay disks available
- Assayed for Abbott CELL-DYN Sapphire, Ruby, 3200 and Manual Methods.
CBC-CAL PLUS
NEK-CAL
The NEK-CAL is designed for calibration of hematology analyzers. Values are provided for WBC, RBC, Hgb, MCV, Hct, and Plt. NEK-CAL has 45-day closed vial with 5-day open vial stability.
Tech-Cal
Tech-Cal is designed for calibration of Bayer ADVIA 120, 2120, 2120i and 60 hematology analyzers. The ADVIA 120, 2120 and 2120i has a bar-coded assay table which includes values for WBCB (WBCP), RBC, Hgb, MCV, CHCM%, Plt, NEUTx%, and NEUTy%. TECH-Cal is bar-coded for correct QC file access. Tech-Cal calibrator has 45-day closed vial with 5-day open vial stability.
- 45-day closed vial stability
- 5-day open vial stability
- Packaged in 3.5mL pierceable screw cap tubes
- Assayed for Bayer Advia 120, 2120, 2120i and 60.
CBC-CAL PLUS is designed for the calibration of BECKMAN COULTER hematology analyzers. Values are provided for WBC, RBC, Hgb, Hct, MCV, Plt, and MPV parameters for DxH and LH Series Diluent systems. CBC-CAL PLUS has 45-day closed vial stability with 7-day open vial stability.
Features
- 45-day closed vial stability
- 7-day open vial stability
- Packaged in 4.5mL pierceable screw cap tubes and 5mL tubes
- Assayed for BECKMAN COULTER DxH 800/600, LH 700 Series and 500 series
CD-Cal is designed for calibration of Abbott CELL-DYN hematology analyzers. Values are provided for WBC, RBC, RBCo, Hgb, MCV, PLTo, PLTi and MPV. CD-Cal has 45-day closed vial stability with 7-day open vial stability.
- 45-day closed vial stability
- 7-day open vial stability
- Packaged in 3mL pierceable screw cap tubes
- Assayed for Abbott CELL-DYN Sapphire, Ruby and 3200
CLINI CHECK
MAST OPTIGEN
- Detection of multiple allergens in single test device(panel format) offers unmatched cost effectivness and convenience.
- Allergens used are well-characterised and standardised, enhance accuracy of final results.
- Rapid test procedure, which shortens the therapeutic turn around time.
- Simple and user friendly procedure decrease dependability on skilled manpower
- Test panel designed with computerised algorithm for fluid dynamic, reduce sample requirement more than 30 allergens, maximises patient comfort during diagnostic process
- Mobile
8806022115
- Address
tuljai Health Care Flat no 607 A-3 Wing, Society Atul Nagar, Warje Pune, Maharashtra 411058